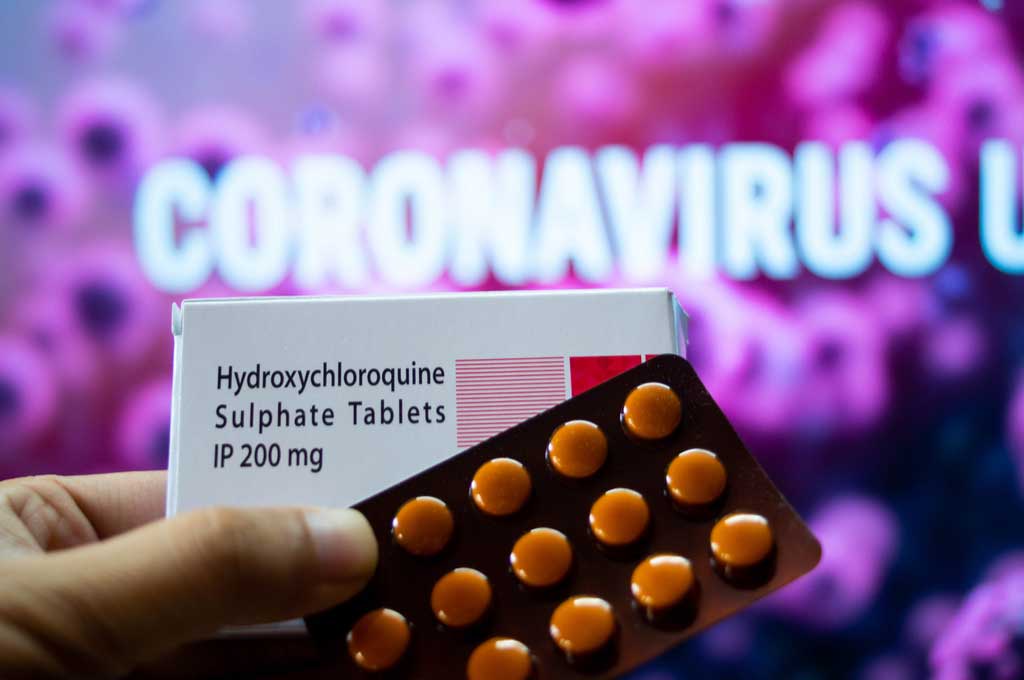
The U.S. Food & Drug Administration (FDA) revoked the Emergency Use Authorization for chloroquine and hydroxychloroquinehydroxychloroquine hy·droxy·chlor·o·quine | \ -ˈklōr-ə-ˌkwēn, -kwin : a drug derived from quinoline that is administered orally in the form of its sulfate C18H26ClN3O·H2SO4 to treat malaria, rheumatoid arthritis, and lupus erythematosus to treat hospitalized COVID-19COVID-19 \ ˈkō-vid-nīn-ˈtēn : a mild to severe respiratory illness that is caused by a coronavirus (Severe acute respiratory syndrome coronavirus 2 of the genus Betacoronavirus), is transmitted chiefly by contact with infectious material (such as respiratory droplets), and is characterized especially by fever, cough, and shortness of breath and may progress to pneumonia and respiratory failure. patients. The agency is ending the emergency use of the antimalarial medication because the risks outweigh any known benefits.
The treatment gained support from the administration in May, including distribution of more than 19 million hydroxychloroquine doses from the National Stockpile to the Department of Veterans Affairs, Department of Defense and several U.S. cities. The drugs failed in several clinical trails and have been found to possibly cause serious heart problems.
The FDA issued the EUA for certain patients with COVID-19 in March. The following month, the FDA and the NIH came out warning against its use.
Studies released in April issued further warnings that HCQ is ineffective as a treatment measure, and one of the studies said HCQ increases the risk of cardiac arrest in COVID-19 patients by more than 2x and was tied to other serious side effects. The drug also did not reduce the need for mechanical ventilation in patients with severe COVID-19 symptoms, nor has it been proven effective as a preventative medicine against coronaviruscoronavirus co·ro·na·vi·rus : any of a family (Coronaviridae) of single-stranded RNA viruses that have a lipid envelope studded with club-shaped projections, infect birds and many mammals including humans, and include the causative agents of MERS, SARS, and COVID-19.
FDA deems Hydroxychloroquine Ineffective, Revokes EUA for COVID-19
The FDA statement says “it is no longer reasonable” to believe the medications are effective for this purpose and that known and potential risks outweigh known and potential benefits. The FDA has determined that chloroquine and hydroxychloroquine “are unlikely to be effective in treating COVID-19 … (and) may cause serious cardiac adverse events and other side effects.”
A 28-day study of 150 COVID-19 patients performed at Shanghai Jia Hong University School of Medicine was halted as a result of findings that patients treated with hydroxychloroquine did not fare any better than patients who did not get the drug.
Here is the FDA’s FAQ on the revocation of its hydroxychloroquine emergency authorization.
The FDA announced the EUA withdrawal in a letter to the Biomedical Advanced Research and Development Authority (BARDA), Office of Assistant Secretary for Preparedness and Response (ASPR), and U.S. Department of Health and Human Services (HHS). Here is the letter explaining the decision to revoke emergency use authorization for HCQ.
Remdesivir, Dexamethasone only available drug treatments for COVID-19
In May, the FDA and NIH approved anti-viral drug remdesivir for treatment of hospitalized COVID-19 patients. The drug, which is being studied in clinical trials globally, is believed to decrease viral RNA production in COVID-19 disease, thus limiting replication and proliferation of the infectioninfection in·fec·tion | \ in-ˈfek-shən a : the state produced by the establishment of one or more pathogenic agents (such as a bacteria, protozoans, or viruses) in or on the body of a suitable host b : a disease resulting from infection.
In the U.K., the NHS this week approved the use of anti-inflammatory drug dexamethasone in the fight against the deadly virusvirus vi·rus | \ ˈvī-rəs : a disease-causing agent that is too tiny to be seen by the ordinary microscope, that may be a living organism or may be a very special kind of protein molecule, and that can only multiply when inside the cell of an organism . The low-dose steroid treatment is part of the world’s biggest trial testing existing treatments to see if they also work for coronavirus.
Dexamethasone cut the risk of death by 1/3 for patients on ventilators, and cut deaths by 1/5 for patients on oxygen. The drug is already used to reduce inflammation in a range of conditions, including arthritis, asthma and some immune systemimmune system : the bodily system that protects the body from foreign substances, cells, and tissues by producing the immune response and that includes especially the thymus, spleen, lymph nodes, special deposits of lymphoid tissue (as in the gastrointestinal tract and bone marrow), macrophages, lymphocytes including the B cells and T cells, and antibodies. and hormone disorders.
It appears to help stop some of the damage that can happen when the body’s immune system goes into overdrive, or cytokine storms, as it tries to fight off coronavirus.

No comments yet. Be the first one to leave a thought.